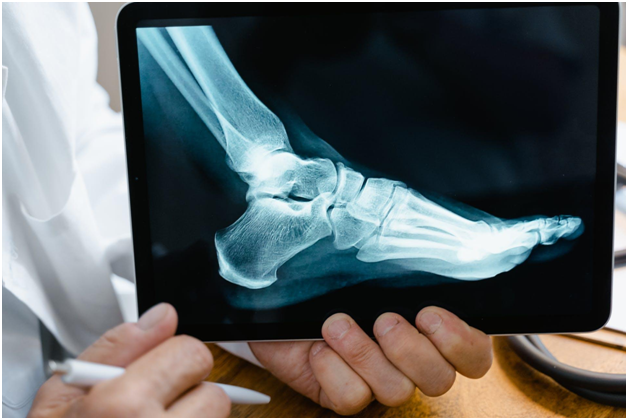
The Cartiva Toe Implant lawsuit has brought to light grave concerns surrounding the functionality and safety of a synthetic cartilage device used to treat arthritis in the big toe. Individuals have reported experiencing pain, requiring additional surgeries, and suffering other injuries due to premature failure of the implant. This has led to a surge in legal action against the manufacturers.
The Cartiva Toe Implant, crafted from polyvinyl hydrogel, is primarily utilized to address arthritis in the big toe, a condition impacting over 2 million people in the U.S. Typically, arthritis results in excruciating pain as cartilage deteriorates, leading to bone-on-bone friction. The Cartiva implant, inserted into the toe joint, was designed as an alternative to traditional fusion surgery, with the aim of reducing pain.
Manufacturers claim the procedure involves minimal tissue removal, resulting in minimal trauma and swift recovery. Ideally, the Cartiva implant replaces degraded cartilage, alleviating painful friction.
However, the Cartiva implant has demonstrated a troublingly high failure rate, often shrinking after implantation, becoming loose, and migrating. This can cause severe pain, nerve damage, and necessitate additional surgeries. The implant has also been associated with infections, fractures, and other complications.
The root cause of the high failure rate appears to be the polyvinyl hydrogel material, which degrades rapidly in some patients, leading to implant shrinkage and migration.
Stryker, a Michigan-based medical device company, manufactures and sells the Cartiva implant. Allegations include claims that Stryker obtained FDA approval by bypassing a full review process, understated the failure rate, and failed to report higher rates to the FDA or recall the implant.
While the official warning label indicates a 13.5% failure rate, subsequent studies suggest it may be much higher, with rates reported as high as 64%. Legal professionals view this as a complex case involving corporate negligence and medical complexities. The outcome could significantly affect Stryker's reputation and finances.
With numerous lawsuits filed and more expected, this case could evolve into a class-action lawsuit. Monitoring its progress is crucial, as it could impact Stryker's future and the market as a whole.
For instance, a single, relatively limited study served as the basis for initial FDA approval, reportedly understating the actual failure rate of the implants. While the official warning label for the Cartiva implant indicates a 13.5% failure rate, subsequent studies suggest that the actual rate of failure may be much higher, with some reporting failure rates as high as 64%. Stryker has been accused of failing to report these higher failure rates to the FDA, nor taking any action to recall the Cartiva implant, despite being aware of the high failure rates.